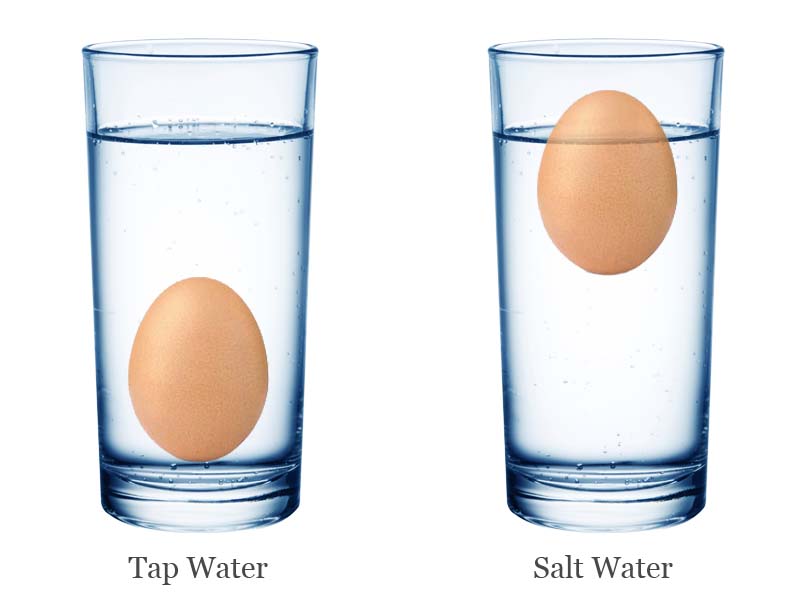
 It is commonly observed that eggs will sink to the bottom of the water when they are placed in ordinary tap water. So, how this happens? And how eggs float in salt water? We will do a simple classic salt water and egg experiment to understand the science behind it.
It is commonly observed that eggs will sink to the bottom of the water when they are placed in ordinary tap water. So, how this happens? And how eggs float in salt water? We will do a simple classic salt water and egg experiment to understand the science behind it.
Precaution: Always wear safety goggles and hand gloves when dealing with chemicals. Also, take the permission from your parents for the experiment, or involve them.
Things You will Need
- Ordinary water
- Table salt
- Tablespoon
- Wide drinking glass
- Egg
What to Do
- Fill the glass with ordinary tap water.
- Place the egg in it, and watch the egg sinking to the bottom of the glass.
- Now take-out the egg from the glass and add 4 to 5 tablespoon full of salt in the water
- Stir the water in the glass to completely dissolve the salt.
- Again, place the egg back into the glass and watch the floating egg.
What is Happening?
When the egg is placed in common tap water it sinks to the bottom, because the density of water is less than the density of egg. But, when you add table salt in the tap water, its density is increased. When the density of water became higher than the density of egg, the egg floated.
Try it
Try to float an egg in the middle of the water.
To do this, follow these steps:
- Fill the glass half-way with water and add 4 tablespoons of table salt, then stir well.
- Fill a cup with ordinary tap water.
- Now, gently add the water from cup to half-filled glass so two layers of different density water don’t mix together.
- Now place the egg in the glass gently.
If two layers of water are not mixed together, the egg will float in the middle of the glass.






